Lean is an interdisciplinary group engaged in developing simple, easily-scalable and environmental friendly strategy to synthesize materials for clean and sustainable energy. Others targets are comprehending fundamental properties such as: i) ion transport in hybrid organic-inorganic polyelectrolyte; ii) electronic transport in ceramics by impedance spectroscopy technique; iii) understanding the charge-transfer at nanomaterials-electrolyte interface with particular interesting in photoelectrochemical cells (PEC) applications.
Thin films in nanoscale for photoelectrochemical (PEC) cells
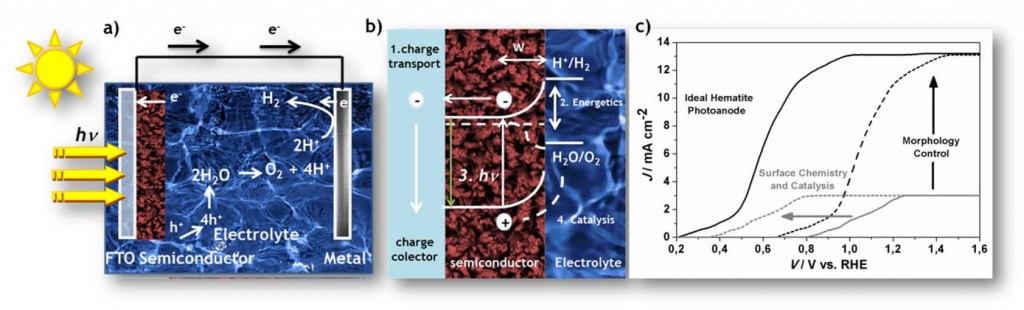
Among the available photoanode materials, the characteristics of hematite (α-Fe2O3) are especially promising. Fe2O3 is abundant, has excellent chemical stability in an aqueous environment and favorable optical band gap. Although attractive, the poor optoelectronic properties necessitate application of large external potential to split water assisted by solar irradiation, limiting the high performance of this material. Nowadays, pure or doped hematite remains as prototype system for PEC devices. Three key challenges need to be addressed to overcome their limitations: i) improving the absorption of light, ii) improving charge separation and, iii) increasing the efficiency of the desired chemical reactions at surfaces. Indeed, it is a consensus in the scientific community that the state-of-art in this field is to find a way to selectively change or overcome one limitation without adversely altering the others. The experimental effort is being focused on developing strategies to reach the perfect harmony between nanostructured morphology with active surfaces for chemical reactions predicted by theoretical calculations (Fig. 1b-c).
Figure: a) Schematic of water splitting reaction using n-type semiconductor as photoanode; b) Details of the charge transfer at photoanode made of hematite; c) Ideal behavior of hematite as photoanode can be achieved combining well controlled morphology for efficient charge transport and active surface for chemical reaction.
We are currently investigating the role of insertion of dopants, undelayer material and surface layer on the conductor substrate and/or photoactive layer in order to improve the photoelectrochemical performance.
We are grateful to the Brazilian agency (FAPESP and CNPq) for support this work
Facile route to synthesize metal oxide nanostructures
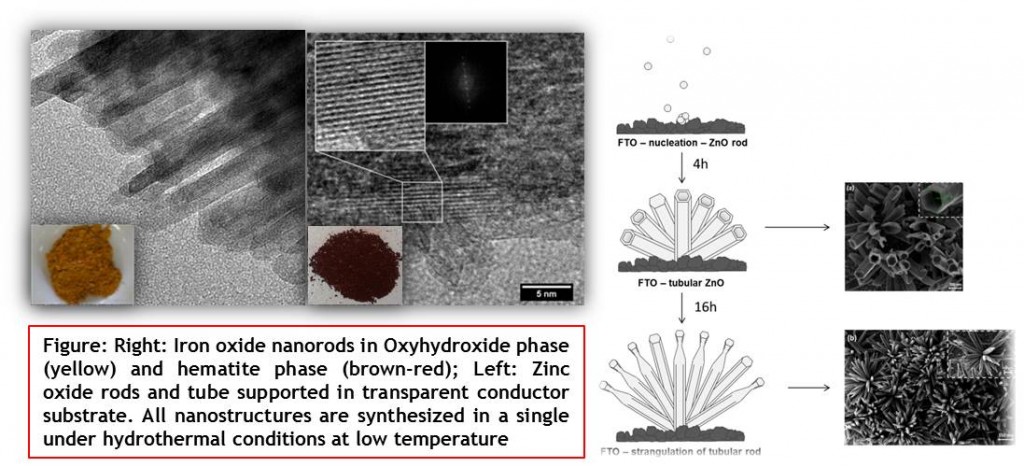
We are focused in developing an effective low cot synthetic route to produce new materials (in nanoscale), easily scalable for applying in alternative energy devices to provide unlimited, clean and carbon free fuels. Additionally, we are interested in exploring and understanding the fundamental properties observed on the obtained materials by these developed methodologies For instance, iron oxide and zinc oxide in nanoscale has been object of intense research because these materials present low cost, mild environmental impact associated to efficient semiconductor properties, which allows it use on several applications. As depicted in the Fig. below we have developed a well-controlled one dimensional nanostructures and investigated the morphology evolution and their optical and structural properties. It was found that the morphologies can be designed during the synthetic process controlling the time and temperature.
We are grateful to the Brazilian agency (CNPq) for support this work
Hybrid organic-inorganic polyelectrolyte and electrochemical devices
Since the first report, in 1973 by P.V. Wright, the interest for replacing liquid electrolyte for ionic conducting polymer membrane was intensified with the extraordinary development of “lithium-ion” batteries that started in the early 1990’s. To make the use of polymer electrolyte possible, in 1998 was proposed the insertion of nanoparticles in the polymer matrix for enhance the ion mobility until then very low. Thereby, the use of solid polymer electrolyte enables the fabrication of flexible, compact devices, free from leaks and available in varied geometries. In 2005 we discovered, by combining theoretical calculation, IR spectroscopy and impedance spectroscopy a novel solid polymer polyelectrolyte whose ion transport mechanism was found to be decoupled to the matrix motion. This find was published in 2005 as communication in the Chemistry of Materials journal and received special interest because we have shown a fast lithium ion motion decoupled of the polymer chain motion, suggesting an efficient conductor membrane for lithium ion battery or smart windows. In 2007 we published the first application of this ion conductor membrane in an electrochromic device with large active area (prototype with 50 and 100 cm2), using WO3 as electrochromic electrode and CeO2-TiO2 as counter-electrode. The performance of this novel polyelectrolyte was published in the Solar Energy Materials and Solar Cells journal and was considered as one of the best study involving polyelectrolyte/performance in the field by the reviewer. Indeed, the device remained working over 90.000 cycles without signal of degradation. Two years later, a group in China using our strategy to produce polyelectrolyte (named as SPHP, single-phase hybrid polyelectrolyte) built electrochromic device showing a great performance, reproducibility and durability. Nowadays, we are interested in understanding the hybrid organic-inorganic matrix developed in order to guarantee higher ionic conductivity and increase the life time of devices performance, using the SPHP as solid electrolyte.
Figure: a) and b) Electrochromic devices with hybrid organic-inorganic polyelectrolyte; c) SPHP doped with iodide (I2) and lithium iodide (LiI) for application in dye sensitizer solar cells.